- /
- Blog / Spectrophotometer & Portable Spectrophotometer: Working Principle and Laboratory Applications
Spectrophotometer & Portable Spectrophotometer: Working Principle and Laboratory Applications

Introduction
A spectrophotometer assists in finding the amount of light an object absorbs at a given wavelength. It can be considered an apparatus that combines a wavelength spectrometer and a photometer—that is, to measure the amount of light entering the object. By measuring absorbance, scientists know the concentration, chemical nature, and optical behavior of most materials. This technique is heavily used in chemistry, biology, environmental science, medicine, and industrial quality control. Portable versions take that idea outside the lab for quick checks.
Principle of Spectrophotometry
The basic concept in spectrophotometry is the Beer-Lambert Law, which includes absorption, concentration, and sample thickness. The basic form of this law states:
A=εcl
Here,
- A is the absorbance,
- ε is the molar absorptivity (a constant for each substance),
- c is the concentration of the solution,
- l is the path length of light through the sample (usually the width of the cuvette).
Another useful relationship is:
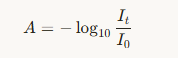
where I0 is the intensity of light entering the sample, and I is the intensity that comes out.
Main Components of a Spectrophotometer
A spectrophotometer consists of the following parts :
- Light source: Common sources are tungsten for visible light and deuterium for UV, as well as xenon and globars in special ranges of lamps. Here, the selection of the light source largely depends on the wavelength of the region.
- Monochromator: It makes use of a limited wavelength range detected through a prism or a diffraction grating. Then it transforms the broad light emitted by the source into a limited light range corresponding to the absorption peak of the sample.
- Cuvette and sample holder: The sample is put in a tiny clear container - usually glass or quartz. For UV work, quartz cuvettes are used because ordinary glass absorbs the UV light. Path length is usually 1 cm - this makes the calculations consistent.
- Detector: The detector helps in the detection of light passing through the sample and its conversion to an electric signal. The detector is normally made up of a photodiode and a photomultiplier tube, which can easily detect changes in the signal intensity.
- Display and data processor: Modern instruments show absorbance and transmittance digitally on a screen and may have a computer attached for data storage, plotting, and analysis.
Some spectrophotometers also split the light into two paths with mirrors and beam splitters. One for the sample & one for the reference. In this setup, error correction for lamp intensity or electronic drift is added.
Working of a Spectrophotometer
The working process is simple. Here are the key steps
- Warm-up and calibration: The light source requires a few minutes to stabilize. In the next step, a "blank" solution - usually the solvent without a sample - is loaded to establish a baseline or zero absorbance.
- Wavelength selection: At first, one selects a wavelength to ensure strong absorption. In the later stages, the monochromator filters it out.
- Light passing: The cuvette allows light of a specific wavelength to enter. The result sample molecules absorb photons, and the remaining ones strike the detector.
- Detection and calculation: Inside the detector, the transmitted light converts into an electrical current. The instrument then calculates absorbance using the formula above.
Some practical distinctions exist between single- and dual-beam systems:
In a single-beam system, one beam of light goes through the blank and then through the sample. Cheaper but more prone to drift when lamp intensity changes during measurement.
In a double-beam system, the beam is split into two parts so it can reach the sample and reference cuvettes simultaneously. This is the more expensive design because it compensates for the variations in the light source.
Different Types of Spectrophotometers
The applications are numerous.
- Quantitative Analysis: This is what labs use to calculate nucleic acid and protein concentrations. It measures solute in mixtures.
- Chemical Analysis: It is used by chemists to distinguish compounds. They look for fingerprint-like absorption peaks.
- Environmental Monitoring: Field agents use it to test water and air quality. It is very useful for pollutant detection.
- Pharmaceuticals: It's used by drug manufacturers for purity checks. It verifies drug stability and consistency of formulation.
- Food and Beverages: It is used for color and additive checking. It is also used for nutrient analysis.
- Research: Scientists use it for kinetics studies. They follow enzyme activity and produce standard curves from known references.
Best Practices for Accurate Readings
You have to follow protocols. Before you run samples through the machine, calibrate it with a blank solution. This is the baseline.
Pick the right container. You need clean quartz or glass cuvette sleeves. For some wavelengths, plastic does not work. You must avoid bubbles in the solution. Fingerprints on the cuvette will also affect readings - hold the cuvette by the frosted edges.
Warm up the instrument. Reproducibility depends on the optics being aligned.
Spectrophotometry Advantages & Limitations
Spectrophotometers offer several benefits:
- They are non-destructive; Most of the samples are recoverable after measuring.
- Measurements are quick - often just a few seconds per sample.
- The method is quantitative and easily reproducible if done right.
- With appropriate accessories, the same instrument can operate in ultraviolet, visible, and infrared ranges
Despite its usefulness, spectrophotometry has some drawbacks:
- Stray light, scattering from dirty cuvettes, and particles in solution increase noise and reduce accuracy.
- In a mixture, overlapped spectra from several components can be hard to interpret without separation or advanced software.
Conclusion
Portable spectrophotometers are fundamental analytical tools. This includes the large benchtop units as well as the smaller portable models. They are needed in modern laboratories. They operate on the Beer-Lambert law principles. It means they can do quantitative and qualitative analysis of many substances.
The fields of use are varied. From environmental testing to drug making, light measurement is important. Proper calibration and sample preparation are very important. Knowing the operational limits improves accuracy. When used right, these tools provide reliability for critical scientific and industrial decisions.
Contact Presto Group Today!
Brand credibility takes a major hit due to color inconsistency. Choose from our range of the latest spectrophotometers for precise color matching and batch-to-batch consistency.
Call us: +91 9210903903
Email: info@prestogroup.com
Visit: www.prestogroup.com
Address: Plot No. I, 42, NH-19, Block C, DLF Industrial Area, Sector 32, Faridabad, Haryana 121003
Recent Blogs
- Spectrophotometer & Portable Spectrophotometer: Working Principle and Laboratory Applications
- Paint Tester & Coating Testing Equipment: Types, Testing Process, and Industrial Applications
- Moisture Meter: Types, Working Principle & Applications in Industrial Labs
- Environmental,Pharmaceutical, and Research Laboratories in BOD Incubator
- Vibration Tables Testing in Automotive and Aerospace Component



